Adrenal Stress Profile – A Comprehensive Tool to Assess the HPA Axis – Stress Response and Resiliency
SPECIMEN TYPE: SALIVA
Only qualified healthcare providers may order laboratory testing.
Why choose Genova Diagnostics’ Hormonal Health Products?
HPA axis dysfunction is seen as a root cause for disease, making evaluation vital to a patient’s overall health.
Genova’s ASP is comprehensive – assessing cortisol diurnal rhythm, DHEA, and optional cortisol awakening response (CAR).
Salivary testing is an easy, non-invasive, at-home collection.
A Comprehensive Tool to Assess the HPA Axis – Stress Response and Resiliency
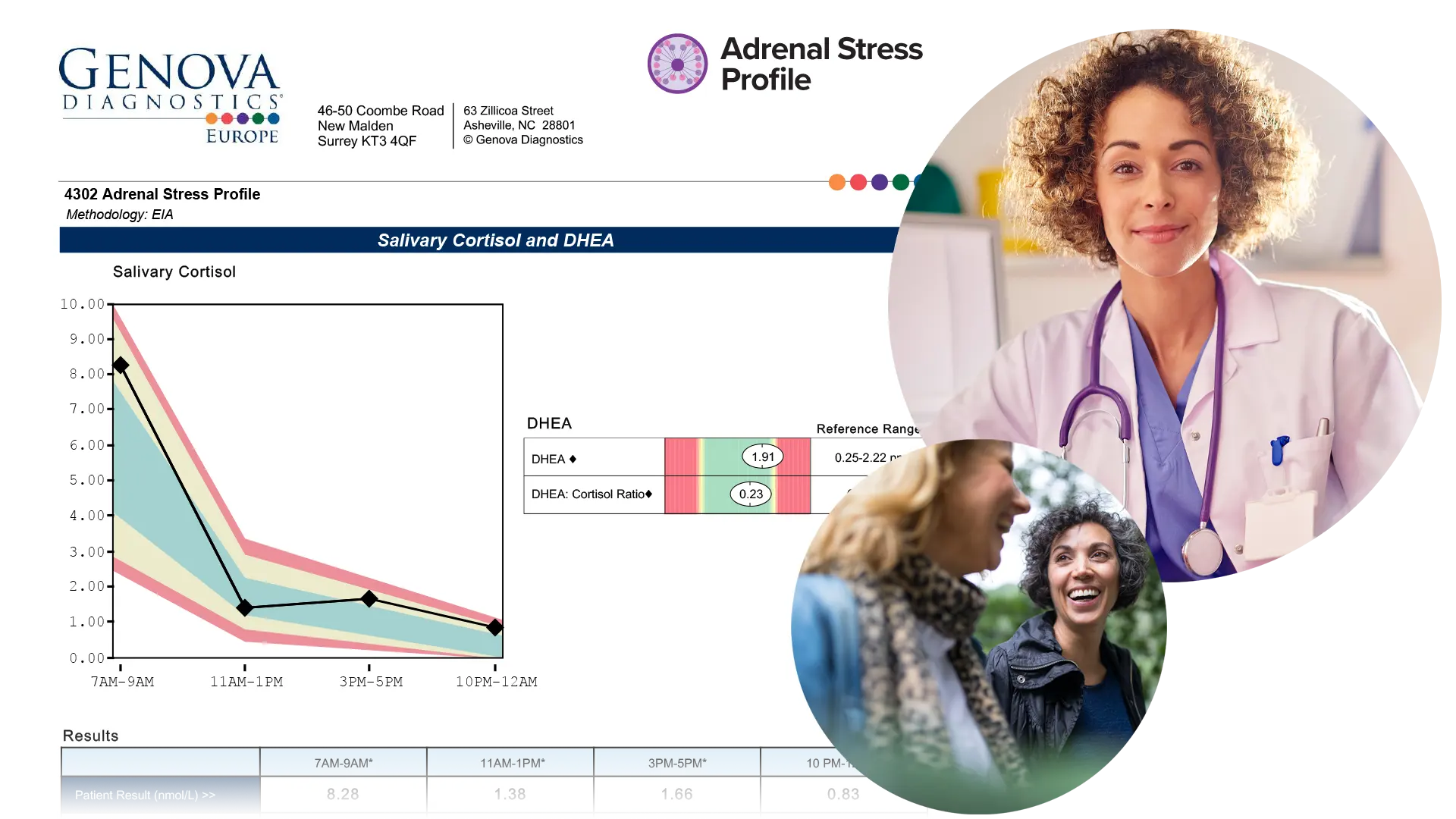
The Adrenal Stress Profile (ASP) provides an assessment of the Hypothalamic-Pituitary-Adrenal (HPA) axis using carefully timed salivary samples of the hormones cortisol and DHEA. Salivary testing is an easy, non-invasive option to measure unbound, biologically active parent hormone levels. The report offers an easy-to-interpret graphic which plots the results of the natural diurnal rhythm.
Which patients might benefit from HPA axis testing?
Daily hassles, chronic pain, blood sugar dysregulation, work stressors, and poor relationship quality can alter the HPA axis. Imbalances in adrenal hormones can have a wide range of negative consequences that can adversely impact a patient’s overall quality of life.
HPA axis dysfunction is associated with many conditions including but not limited to:1,2,3,4,5,6,7,8,9
- Hypertension
- Cardiovascular disease
- Gastrointestinal and immune dysregulation
- Diabetes and metabolic syndrome
- Depression
- Chronic fatigue
- Insomnia
- Weight gain
- Persistent pain
- Neurodegenerative disease and cognitive decline
About the Adrenal Stress Profile
- Four cortisol samples measured throughout the day give insight into the natural circadian diurnal cortisol rhythm, and help clinicians address specific daily stressors.
- DHEA is measured once in the 7:00 AM – 9:00 AM sample.
- Ratio of DHEA to cortisol is calculated to provide insight into anabolic/catabolic balance.
Optional Add-on:
- Cortisol Awakening Response (CAR)
- Two awakening samples to evaluate CAR.
- CAR is a transient, immediate rise in cortisol upon awakening and is distinct from the diurnal rhythm. CAR reflects a person’s ability to cope with anticipated challenges and their perception of control around chronic stress, providing insight into HPA axis resiliency.
- Salivary secretory IgA (sIgA)
- sIgA is secreted across mucosal membranes and regulates immune function
- sIgA levels and immunity can be impacted by stress
Adrenal Stress Profile testing can reveal these HPA axis imbalances and provide direction for clinical intervention with targeted therapeutic treatments such as nutrient support and/or adaptogens, stress management, behavioral modification and lifestyle interventions.
In response to stress, your adrenal glands produce hormones that can have a negative impact on your overall health.
- There are many conditions associated with adrenal imbalances:
- High blood pressure
- Cardiovascular disease
- Gastrointestinal and immune issues
- Diabetes and metabolic syndrome
- Depression
- Chronic fatigue
- Sleep issues
- Weight gain
- Persistent pain
- Nervous system diseases
- Memory and thinking issues
Adrenal Stress Profile testing can reveal adrenal hormone imbalances and can help your practitioner create a therapeutic plan. Patients often report having more energy, better sleep, improved mood, and clearer thinking when adrenal imbalances are corrected.
The Adrenal Stress Profile requires 4-6 saliva samples throughout the day. We have many resources to help make your testing experience a success. Review the Test Preparation tab to learn more about the collection process.
Preparing for this Test
Important Considerations Before Testing
Certain medications and supplements may impact test results, though in some instances that impact is unknown. There are many substances that influence cortisol and DHEA levels, however no known substances will interfere with the ability to run the assay itself.
Genova never recommends that patients discontinue medically necessary medications or supplements in order to complete testing.
There may be times when a patient may stay on a medication or dietary supplement during testing in order to evaluate its effectiveness or impact. A clinician may choose to discontinue a substance in order to evaluate the patient’s baseline. The timeframe to discontinue varies for each substance and is at the discretion of the clinician.
Any questions regarding a medication or supplement impact on biomarker results can be researched by contacting the medication/supplement manufacturer and/or searching the literature (PubMed, Google Scholar) for relevant information. Drug databases such as drugs.com, rxlist.com, or Epocrates may provide additional information.
Glucocorticoids
Any steroid-based preparation including oral, topical (patch, cream), eye drops (minimal impact), nasal sprays, injections, and inhalers may influence the Adrenal Stress Profile findings.1-11 The degree of impact depends on numerous factors including, but not limited to, preparation, specific drug, dosage, chronicity of use, and individual response to medications. Not all glucocorticoids impact results the same.7
In general, exogenous glucocorticoids – even with one-time administration – are known to suppress the HPA axis via negative feedback, which can result in lower endogenous production of cortisol.3,12,13 Long-term use of glucocorticoids can lead to adrenal atrophy, which is slowly reversible.12,13 Glucocorticoid-induced adrenal insufficiency may last up to 2-4 years after glucocorticoid withdrawal.14
When to test a patient following glucocorticoid discontinuation is dependent on multiple factors (mentioned above) and is at the discretion of the clinician. For example, if a clinician wishes to test a patient’s baseline HPA axis function following a steroid injection, it may take up to 4 weeks or longer for recovery to baseline.6,8 A literature search may provide further insight into the clearance rate and impact the particular glucocorticoid may have on salivary testing.
The cortisol assay can cross-react with various glucocorticoid drugs. For example, there is a strong cross-reactivity with the antibody used in this assay and dexamethasone which may result in a false elevation of cortisol. It is unknown how much of the reported result is the patient’s endogenous cortisol, versus how much is cross-reactivity from the medication.
Genova’s hormone assays measure endogenous hormones as well as bioidentical hormone replacement, but not synthetic hormones, due to their different molecular structure. Hydrocortisone is bioidentical cortisol and is prescribed for patients with adrenal insufficiency. Some studies suggest utility of salivary cortisol measurements for monitoring glucocorticoid replacement therapy, however studies are mixed.15
Shift Workers and Alternate Sleep-Wake Schedules
The reference ranges for the Adrenal Stress Profile (ASP) were designed for patients with normal sleep cycles and referenced for the specific time frames as graphed on the test report. However, some clinicians choose to test individuals with altered sleep cycles, knowing that the graph, and reference ranges may not directly apply. Even though the reference ranges may not apply, there is still some clinical information to be gleaned based on the overall slope of the diurnal curve. It is unknown whether extrapolating from the diurnal curve and reference ranges would apply to someone on an alternate schedule – “healthy” or “normal” has not been defined for this population.16-19 If the clinician chooses to test, the clinician can change the morning peak time to the patient’s waking time (within one hour of waking) and then each additional sample will be approximately four hours after the prior.
In order to determine cortisol awakening response (CAR), samples must include both the waking and 30-minute samples. The timing of these collections should reflect when the patient wakes to begin their day. It has been shown in literature that patients who have alternate work schedules may ‘reset’ their internal circadian rhythm and, therefore, CAR sampling can be done during their normal waking cycle. When the patient wakes, have them immediately give a saliva sample, then another exactly 30 minutes later for CAR. These two samples are used for the CAR calculation. The third sample should be within that hour, then every four hours after that to complete the ASP portion.
Bleeding Gums
Samples visibly contaminated with blood should be recollected. This can result in a false elevation of hormones. Blood concentrations of steroid hormones are several-fold higher than saliva levels.20,21 For this reason, brushing and flossing is discouraged for 1 hour prior to salivary collection. Additionally, if the patient has a condition that causes gums to bleed easily, such as gingivitis or periodontitis, or has dentures and other oral appliances, a plan should be in place to ensure gums do not bleed during testing.
Paediatric Patients
The reference ranges for the Adrenal Stress Profile are based off a healthy cohort of patients aged greater than 18. Genova does not have pediatric reference ranges. DHEA is an androgen and is typically lower in prepubescent populations. A literature search may provide further insight into pediatric reference ranges for cortisol and DHEA.
REFERENCES
- Pandya D, Puttanna A, Balagopal V. Systemic effects of inhaled corticosteroids: an overview. The Open Resp Med J. 2014;8:59-65.
- Sastre J, Mosges R. Local and systemic safety of intranasal corticosteroids. J Invest Allergol Clin Immunol. 2012;22(1):1-12.
- Baptist AP, Reddy RC. Inhaled corticosteroids for asthma: are they all the same? J Clin Pharm Therap. 2009;34(1):1-12.
- Stout A, Friedly J, Standaert CJ. Systemic Absorption and Side Effects of Locally Injected Glucocorticoids. PM & R : J Injury Function Rehab. 2019;11(4):409-419.
- Masharani U, Shiboski S, Eisner MD, et al. Impact of exogenous glucocorticoid use on salivary cortisol measurements among adults with asthma and rhinitis. Psychoneuroendocrinology. 2005;30(8):744-752.
- Habib GS. Systemic effects of intra-articular corticosteroids. Clinical Rheumatol. 2009;28(7):749-756.
- Broersen LH, Pereira AM, Jørgensen JO, Dekkers OM. Adrenal Insufficiency in Corticosteroids Use: Systematic Review and Meta-Analysis. J Clin Endocrinol Metab. 2015;100(6):2171-2180.
- Chon JY, Moon HS. Salivary cortisol concentration changes after epidural steroid injection. Pain Phys. 2012;15(6):461-466.
- Silver S, Tuppal R, Gupta AK, et al. Effect of calcipotriene plus betamethasone dipropionate topical suspension on the hypothalamic-pituitary-adrenal axis and calcium homeostasis in subjects with extensive psoriasis vulgaris: an open, non-controlled, 8-week trial. J Drugs Dermatol. 2013;12(8):882-887.
- Krupin T, Mandell AI, Podos SM, Becker B. Topical corticosteroid therapy and pituitary-adrenal function. Arch Ophthal. 1976;94(6):919-920.
- Sandhu SS, Smith JM, Doherty M, James A, Figueiredo FC. Do topical ophthalmic corticosteroids suppress the hypothalmic-pituitary-adrenal axis in post-penetrating keratoplasty patients? Eye. 2012;26(5):699-702.
- Ambrogio AG, Pecori Giraldi F, Cavagnini F. Drugs and HPA axis. Pituitary. 2008;11(2):219-229.
- Lipworth BJ. Systemic adverse effects of inhaled corticosteroid therapy: A systematic review and meta-analysis. Arch Int Med. 1999;159(9):941-955.
- Pelewicz K, MiÅkiewicz P. Glucocorticoid Withdrawal-An Overview on When and How to Diagnose Adrenal Insufficiency in Clinical Practice. Diagnostics. 2021;11(4).
- Jung C, Greco S, Nguyen HH, et al. Plasma, salivary and urinary cortisol levels following physiological and stress doses of hydrocortisone in normal volunteers. BMC Endo Dis. 2014;14:91.
- Cannizzaro E, Cirrincione L, Mazzucco W, et al. Night-Time Shift Work and Related Stress Responses: A Study on Security Guards. Int J Environ Res Public Health. 2020;17(2).
- Lindholm H, Ahlberg J, Sinisalo J, et al. Morning cortisol levels and perceived stress in irregular shift workers compared with regular daytime workers. Sleep Dis. 2012;2012:789274.
- Li J, Bidlingmaier M, Petru R, Pedrosa Gil F, Loerbroks A, Angerer P. Impact of shift work on the diurnal cortisol rhythm: a one-year longitudinal study in junior physicians. J Occup Med Toxicol. 2018;13:23.
- Bostock S, Steptoe A. Influences of early shift work on the diurnal cortisol rhythm, mood and sleep: within-subject variation in male airline pilots. Psychoneuroendocrinology. 2013;38(4):533-541.
- Hofman LF. Human saliva as a diagnostic specimen. J Nutr. 2001;131(5):1621s-1625s.
- Granger DA, Taylor MK. Salivary bioscience: foundations of interdisciplinary saliva research and applications. Springer Nature; 2020.
| Collection Pack Instruction | Recommended Timeframe to Discontinue | Possible Impact on Results |
|---|---|---|
| Colonoscopy or barium enema | 4 weeks | May alter bacteria levels; 4 weeks is thought to be enough time for the intestinal flora to reestablish a baseline and for the GI tract to normalize after these procedures. |
| Antibiotics | The North American SIBO Consensus group recommends discontinuing antibiotics 4 weeks prior to testing. This may be beneficial for initial testing.1 Clinicians may choose to test shortly after cessation of antibiotic therapy to confirm eradication. | |
| Antifungals, herbal/natural antimicrobial products | 2-4 weeks | Can alter/influence bacterial composition. |
| Pepto-Bismol | Generally given as part of H.pylori treatment, Pepto-Bismol is also known to impact other bacteria.2 | |
| Laxatives, stool softeners, stool bulking agents (Ex-Lax, Colace, Metamucil, Fibercon) | 7 days | The North American SIBO Consensus group recommends discontinuing promotility drugs and laxatives 7 days prior to testing only if tolerated by the patient. A 4-week gap had previously been recommended, but the consensus group agreed that this time frame may not be practical for discontinuation.1 These substances can result in faster transit time and an earlier delivery of the lactulose substrate to the colon, resulting in a false-positive finding. If the use of laxatives normalizes transit time, continuing the medication may not be an issue. Fiber-containing agents may feed large intestine bacteria resulting in a false-positive finding. In patients who are severely constipated, it may be difficult to be off of this support for 7 days, so some clinicians may choose to discontinue at least 2-4 days prior. Other ways to help support patients with constipation include exercise, appropriate hydration, and trying to avoid foods that the patient knows aggravates constipation. |
| Antacids containing aluminum or magnesium hydroxide (For collections based in the United States, examples include Maalox liquid, Equate, Milk of Magnesia, Rolaids, Mylanta) | Certain antacids can influence transit time, which may influence test results. | |
| Diet must be limited; the only ALLOWED foods include baked or broiled chicken, fish or turkey (salt and pepper only), white bread (only), plain steamed white rice, eggs, clear chicken or beef broth (no vegetable pieces). Allowed beverages include water, plain coffee and tea (no sugar/artificial sweeteners or cream). | 24 hours before | High fiber foods or foods containing fermentable carbohydrates can be acted on by the large intestine bacteria. In order to ensure the test does not result in false positives or an elevated baseline from large intestine bacteria, the recommended diet must be followed. Clinicians may extend the diet to 48 hours for patients who are constipated. |
| Probiotics (i.e. acidophilus) | Probiotics have been shown to affect hydrogen levels on breath testing; the North American SIBO Consensus group did not reach a firm position statement on stopping probiotics prior to breath testing.1 | |
| Fast from food, only water is allowed | 12 hours before | Fasting prior to breath collection is important to ensure that the small intestine is clear of any food. A false positive or elevated baseline may result from not adhering to this instruction. |
| No non-essential medications or supplements, gum, candy or mouthwash | May result in elevated breath gas levels and possibly false-positive results. | |
| No smoking (including secondhand), sleeping, or vigorous exercise; this includes waiting at least 1 hour after waking for the day | 1 hour before and during | Results in elevated breath gas levels and possibly false-positive results.1 |
| Toothpaste | Toothpaste may contain fermentable ingredients for oral bacteria, resulting in a false-positive test result. |
We do not suggest collecting during an acute gastrointestinal infectious illness. Transit time and intestinal flora may be altered, which can impact test results.
Patients with Lactose Intolerance or Allergy to Lactulose
This test uses lactulose as its testing agent, and is not recommend for individuals who have had allergic reactions to lactulose, or are on a galactose/lactose-restricted diet. The full dose of lactulose for this test is 10 grams. Allergic reactions to lactulose, which are IgE-mediated and may present with such symptoms as hives, difficulty breathing, or swelling, are quite rare but can be serious. More commonly individuals may have a food sensitivity, which involves a milder, delayed reaction, which can include various symptoms including congestion, gastrointestinal discomfort, and eczema. It is also worth noting that the lactose intolerance precaution refers to those individuals who may simply have symptoms of bloating or discomfort after consuming lactose due to lactase enzyme deficiency. Though not likely dangerous, GI distress is possible with exposure to this drink in these individuals. Lactose intolerance will not interfere with test results. The healthcare provider will need to decide whether to run the test in light of a possible symptomatic response.
Patients with Diabetes
The test uses lactulose as its testing agent, and should be used with caution in diabetics, as it has the potential to raise blood sugar. Per the VistaPharm, Inc. lactulose package information,3 “since lactulose solution contains galactose and lactose it should be used with caution in diabetics.”
Transit Time
The normal transit time of lactulose (10 g) in healthy fasting patients, from the mouth to the junction between the small and large intestine (oro-cecal transit time, or OCTT), is approximately 90 minutes. In general, transit times have been found to vary in humans. Given such findings, transit time should be taken into consideration when interpreting breath testing. Substances meant to alter transit time, such as laxatives or prokinetics, should be discontinued 7 days prior to testing. If the patient is constipated, the clinician may extend the length of the limited diet prior to testing, from 24 hours to 48 hours.
Special Instructions for Patients Weighing 100 Pounds or Less/ Paediatric Patients
Follow the instructions on the blue bag for rolling and stapling the bag in accordance with weight. (Note: stapling will not damage the bag or affect the results.) This ensures that air is being collected from the appropriate part of the lungs.
According to Quintron, the manufacturer of the SIBO collection kit, the test is not appropriate for children under 25 pounds. Much of the testing requires strict adherence to collection instructions, which can be a challenge in pediatrics. There is also a strict dietary restriction in the 24 hours prior to testing regarding avoidance of certain foods that may alter the results of the test. It is required that a patient completely fast, with the exception of drinking water, in the 12 hours prior to testing. This may not be amenable in very small children. The package instructions direct the patient to stir the 10 grams (15ml or 3.3g/5ML) of lactulose solution in 8 ounces/240ml of water and drink that solution within 5 minutes after the baseline breath collection. There is not an adjusted dosage for children.
Additionally, bowel frequency and the pediatric microbiome change drastically in the first few years of life. Because of this, research is still developing on how this drastic change may influence the potential development of SIBO, as well as the most effective means to evaluate and diagnose this population. A gold standard testing option for the evaluation of bacterial overgrowth in pediatric populations has yet to be established. However, there is some literature to suggest using breath test measurement to evaluate SIBO in pediatrics.4
If a patient is not a good candidate for taking the SIBO breath test, you may consider utilising the GI Effects Comprehensive stool profile or the Comprehensive Digestive Stool Analysis 2.0 stool profile to glean information about the GI tract. Although these tests are not diagnostic for SIBO, certain biomarkers may suggest SIBO.
REFERENCES
- Rezaie A, Buresi M, Lembo A, et al. Hydrogen and Methane-Based Breath Testing in Gastrointestinal Disorders: The North American Consensus. Am J Gastroenterol. May 2017;112(5):775-784.
- Pitz AM, Park GW, Lee D, et. al. Antimicrobial Activity of Bismuth Subsalicylate on Clostridium difficile, Escherichia coli O157:H7, Norovirus, and Other Common Enteric Pathogens. Gut Microbes. 2015;6(2):93-100.
- DailyMed Lactulose. National Institutes of Health U.S. National Library of Medicine. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7016bd71-c667-46fc-8c56-5023682e8bbe
- Malik BA, Xie YY, Wine E, Huynh H. Diagnosis and Pharmacological Management of Small Intestinal Bacterial Overgrowth in Children with Intestinal Failure. Can J Gastroenterol. 2011;25(1):41-45.
Support Materials
-
Collection Instructions
Sample Report
Support Guide
How it Works
Consult Healthcare Provider
Your provider will discuss your symptoms and help decide which test is right for you.
Many specimen collections can be completed from the privacy of your home.
Collect Samples
Use a calendar to plan for your collection.
Follow instructions carefully and be sure to add important details about you and your specimens where indicated.
Ship to Lab
Ship specimens using the materials provided.
Schedule time with your healthcare provider to review results and create a plan for your health.
FAQ
- Review information on the Test Preparation tab above for details on how medications and supplements may impact this test.
- Support guides, charts, and additional aids can be found on the Support Materials tab. Additional educational materials can be found in our Learning Library.
RELATED PRODUCTS
Looking for added insight?
Symptoms of conditions can overlap. Certain disease states can influence other body systems. Additional testing can help identify those abnormalities.

The One Day Hormone Check™ is a convenient salivary hormone test that evaluates unbound, bioavailable hormone levels. Hormone testing can help clinicians customise and monitor therapies.

The NutrEval® FMV uses blood and urine to evaluate over 125 biomarkers and assess the body’s functional need for 40 antioxidants, vitamins, minerals, essential fatty acids, amino acids, digestive support, and other select nutrients.


